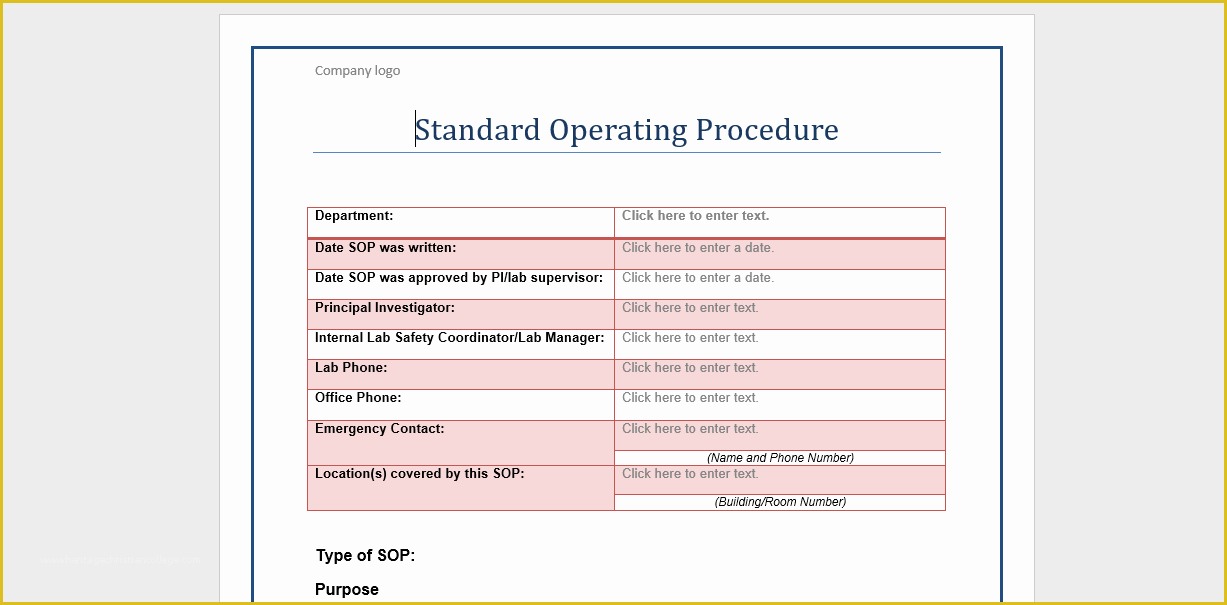
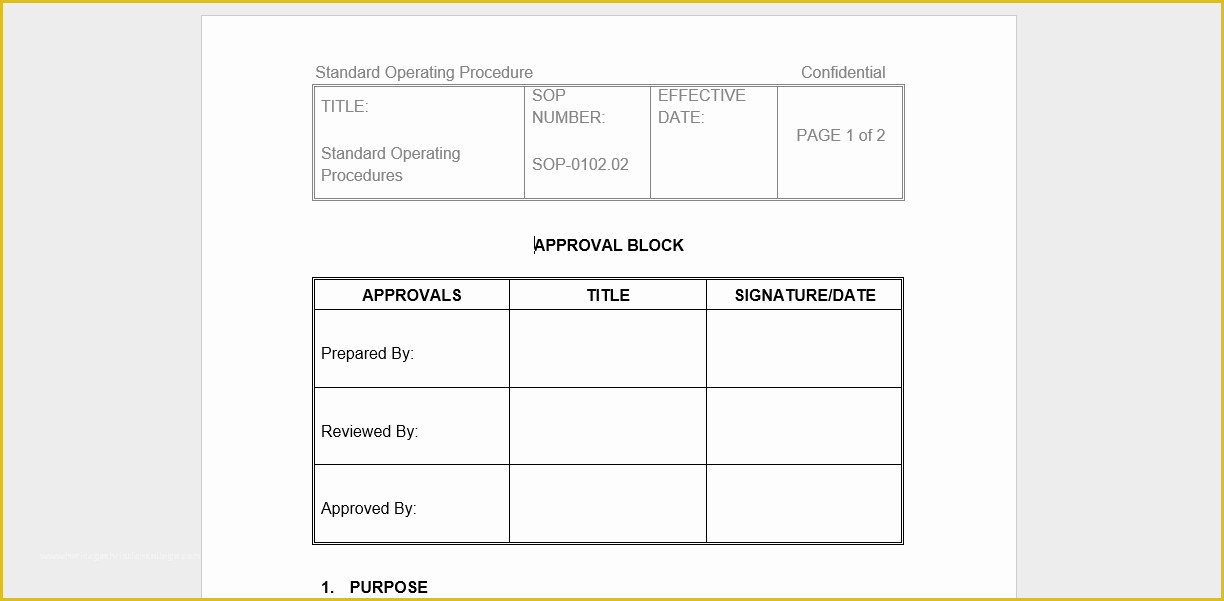
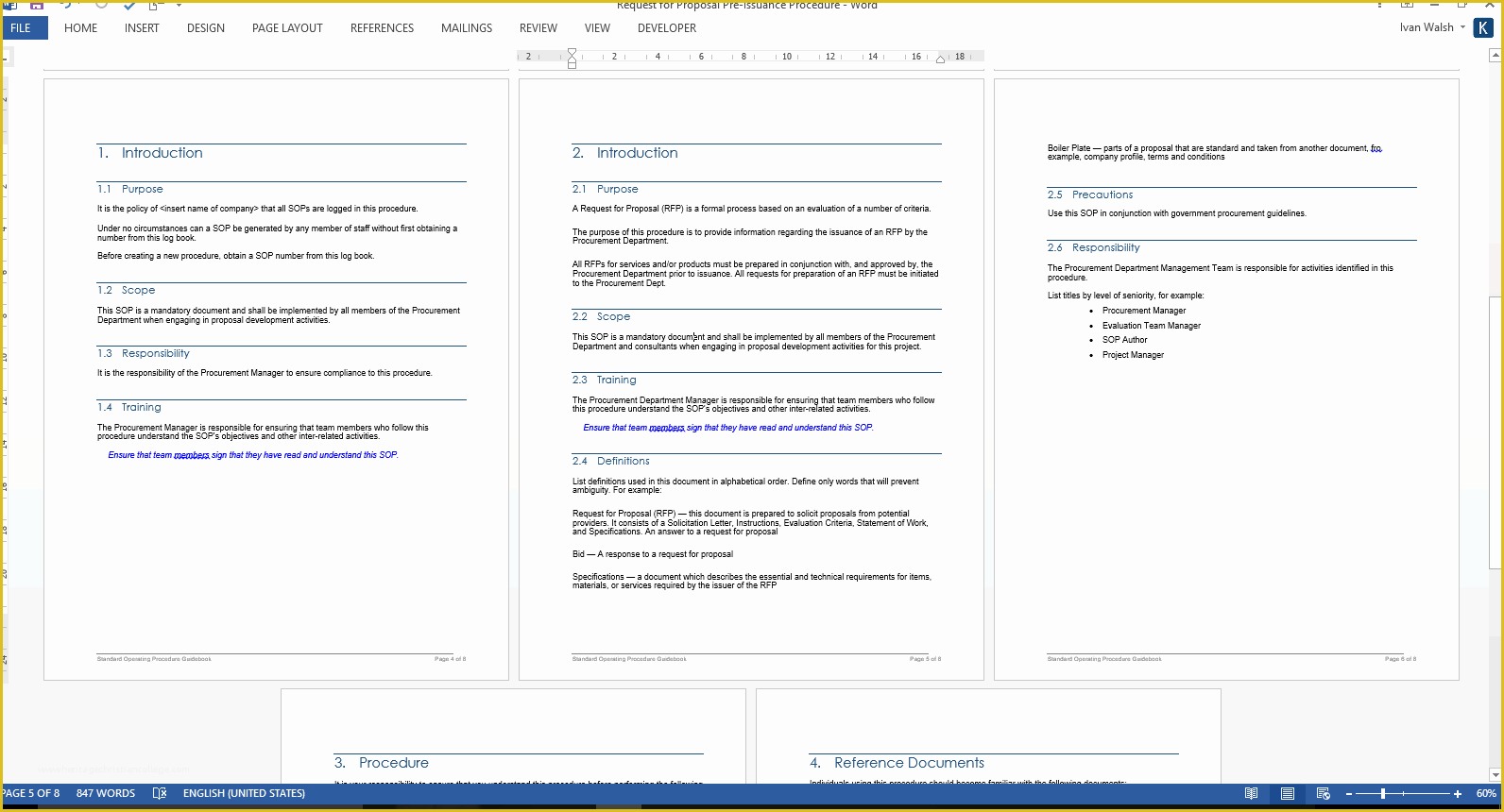
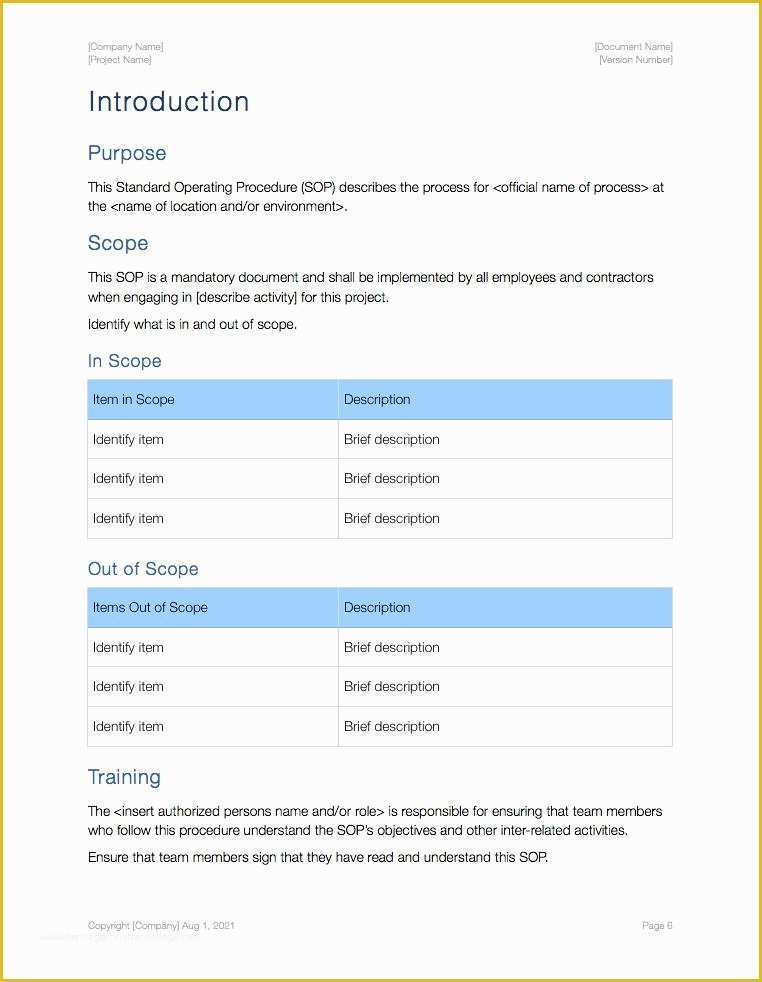
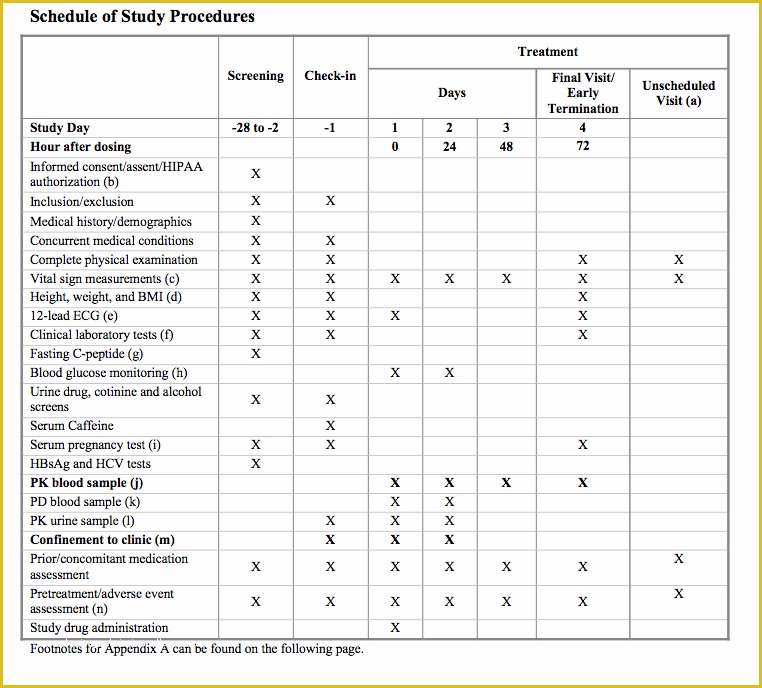
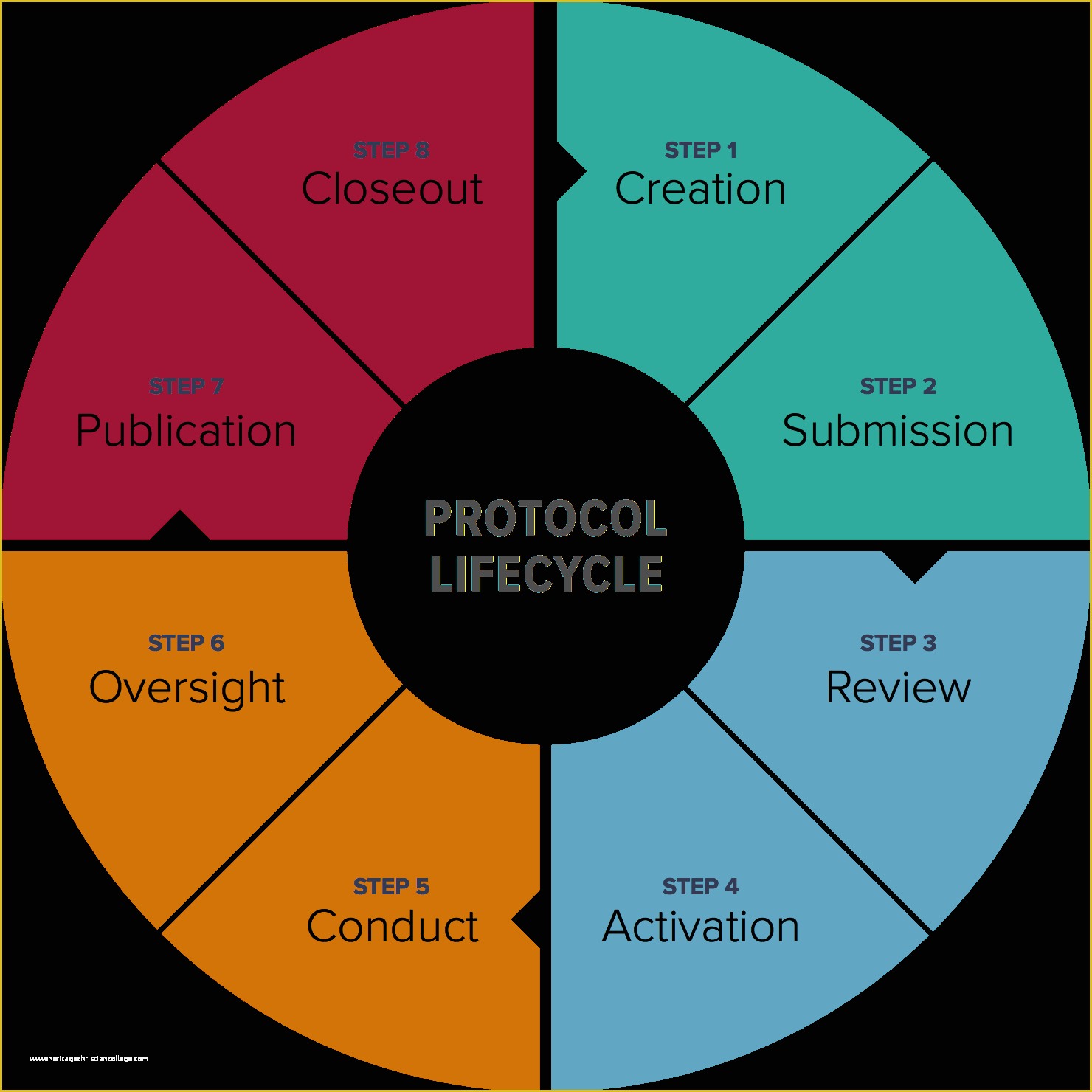
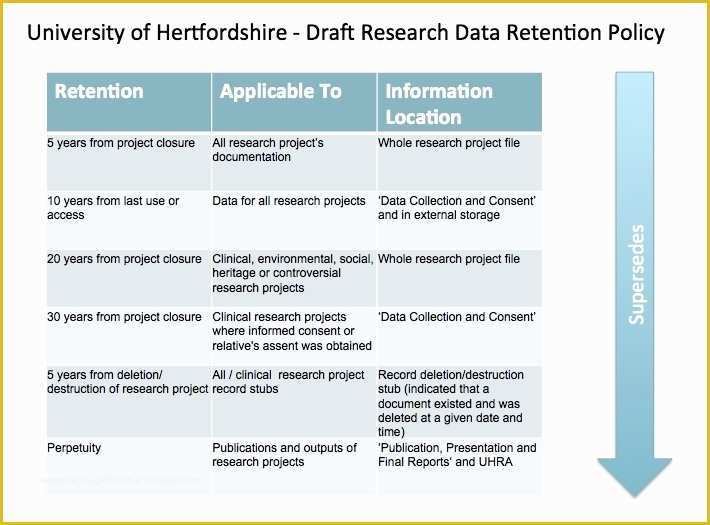
Standard Operating Procedure SOP Template User Guide 36 Page Standard Operating Procedure SOP Template MS .
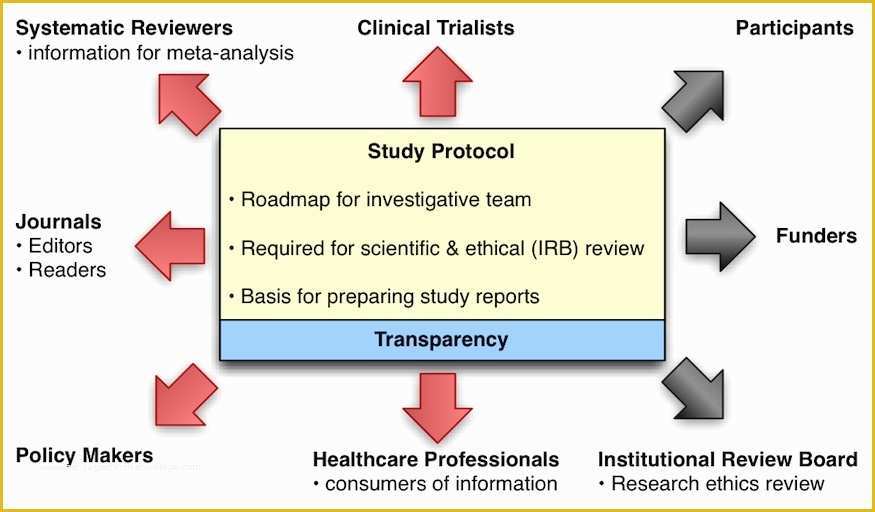
clinical trial clinical trials are experiments or observations done in clinical research such prospective biomedical or behavioral research stu s on human participants are designed to answer specific questions about biomedical or behavioral interventions including new treatments such as novel vaccines s tary choices tary supplements and standard operating procedure template & sop format get free templates and learn from industry practitioners and experts how standard operating procedure sop is used as a pliance tool research dynamics consulting group resdyncg latest news april 2 2015 – webinar on quality control and your clinical trial lorraine will be providing a free webinar on “building quality control into your clinical trial” on thursday april 2 2015 at 3pm edt 607 free resume templates [download ready made samples resume templates [ready made] get hired for a job position by crafting and sending a well formatted resume to an employer download our free premium resume templates that are ready made and professionally designed consort wel e to the consort website consort stands for consolidated standards of reporting trials and en passes various initiatives developed by the consort group to alleviate the problems arising from inadequate reporting of randomized controlled trials free css premium css templates if you can t find a free css website template that suits your needs then why not take a look at the premium templates here ukzn research fice biomedical research ethics all health biomedical and social research at the university of kwazulu natal requires prior ethical clearance by the biomedical research ethics mittee brec pharmacircle no session pharmacircle is an innovative knowledge management pany specializing in the delivery pharmaceutical and biotechnology fields the current clients of pharmacircle™ vary from world leaders to start up panies in the pharmaceutical biotechnology and delivery fields guide for authors clinical therapeutics issn 0149 2918 clinical therapeutics provides peer reviewed rapid publication of recent developments in and other therapies as well as in pharmacoeconomics health policy treatment out es and innovations in and biologics research specialized center of research program specialized center of research program opening soon supports teams of researchers representing different disciplines and engaged in collaborative efforts to discover new approaches to treat patients with blood cancers
clinical research ,