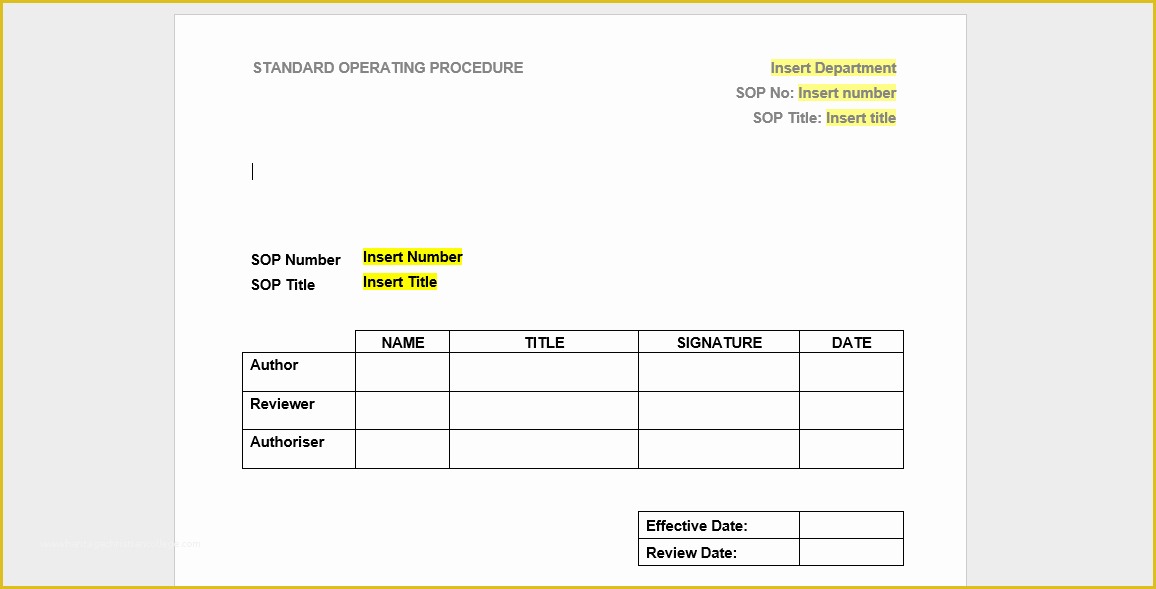
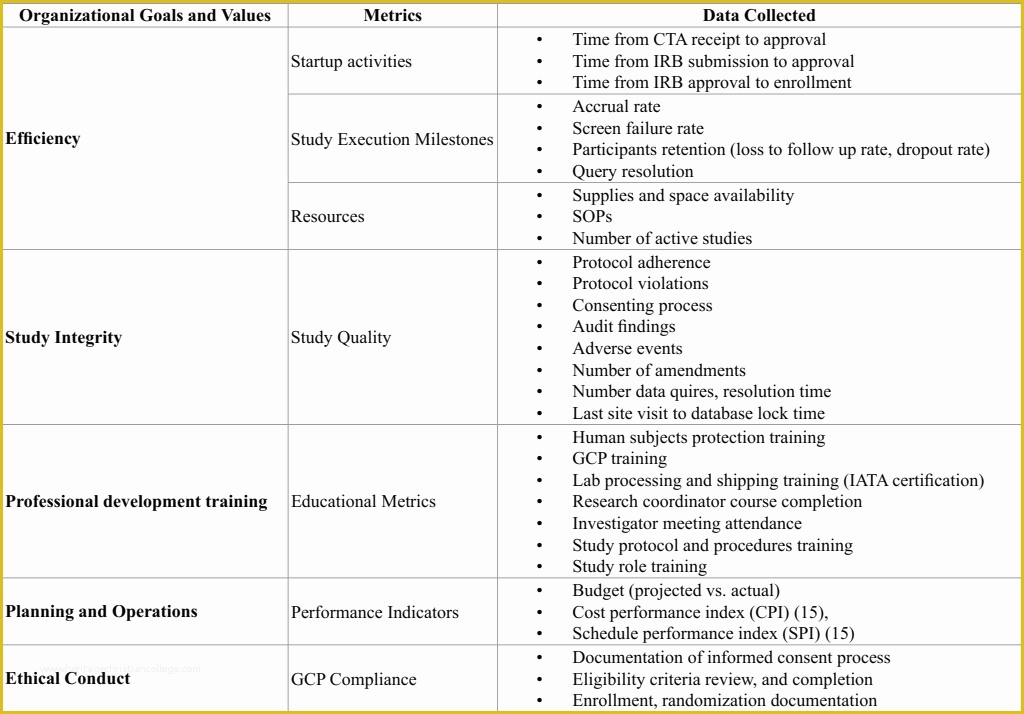
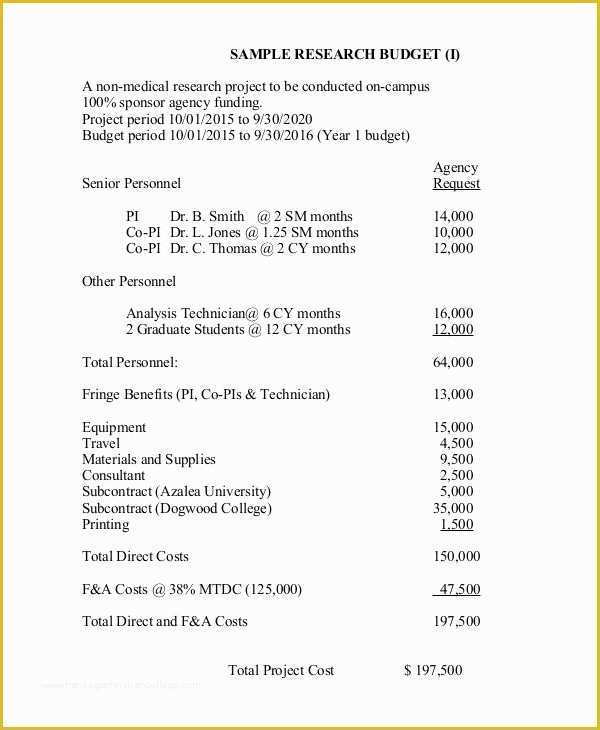
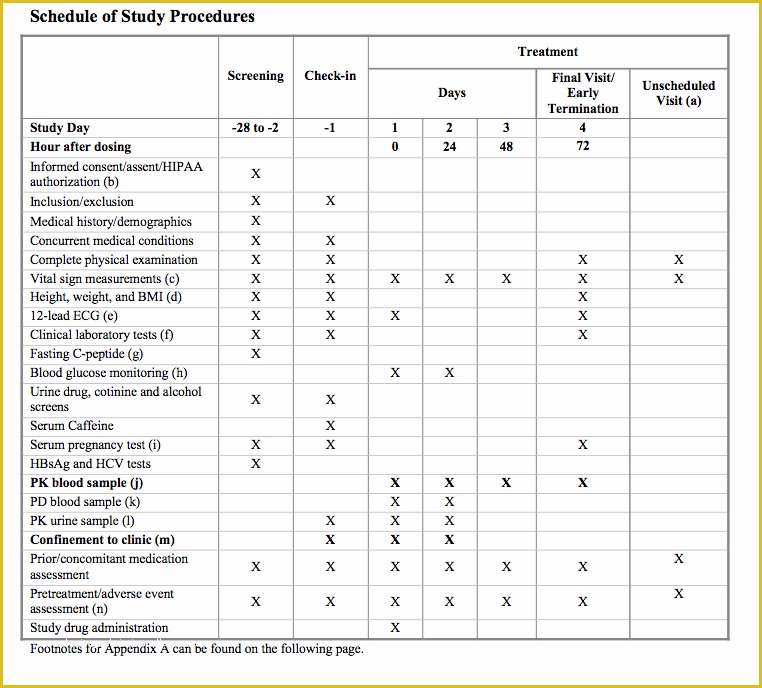
Looking For Sample Medical Business fice Procedure Manual Standard Operating Procedure SOP Template User Guide Clinical Trial Protocol Template Ich Templates Resume .
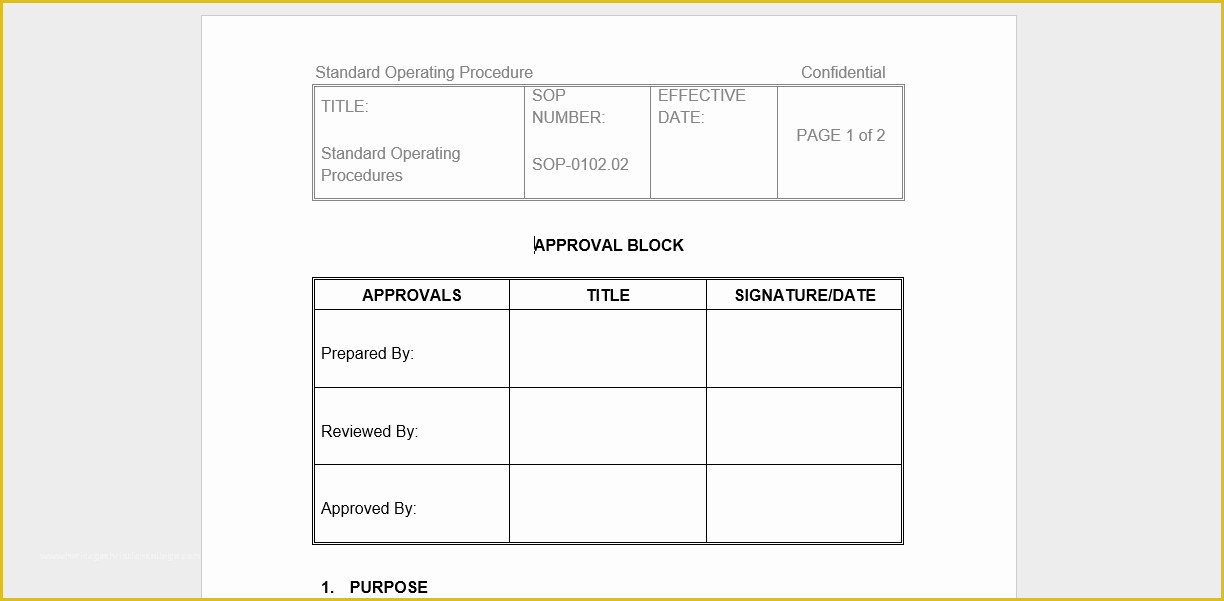
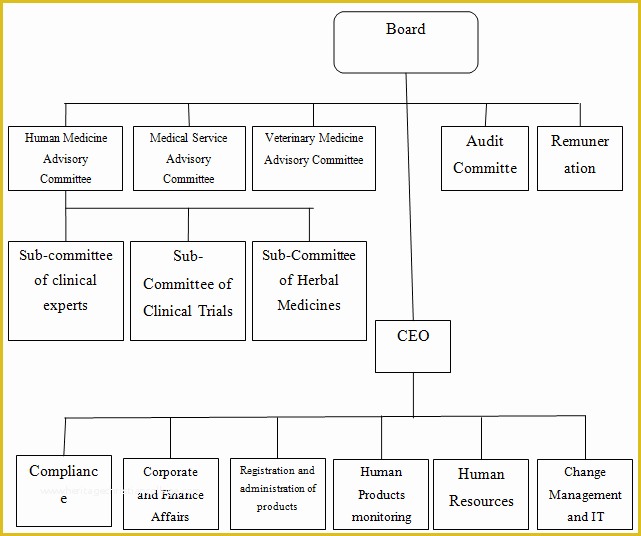
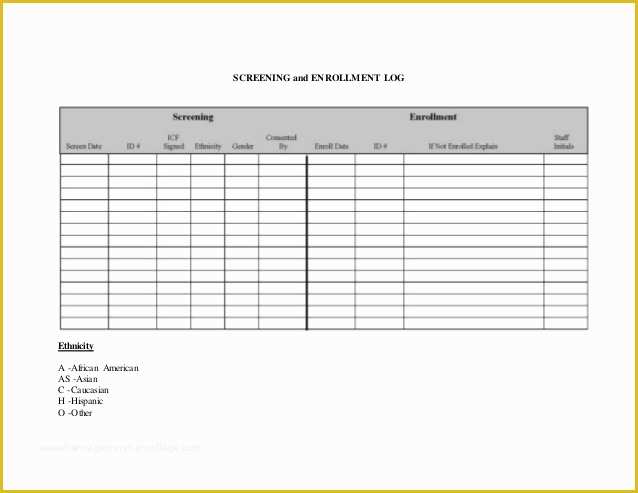
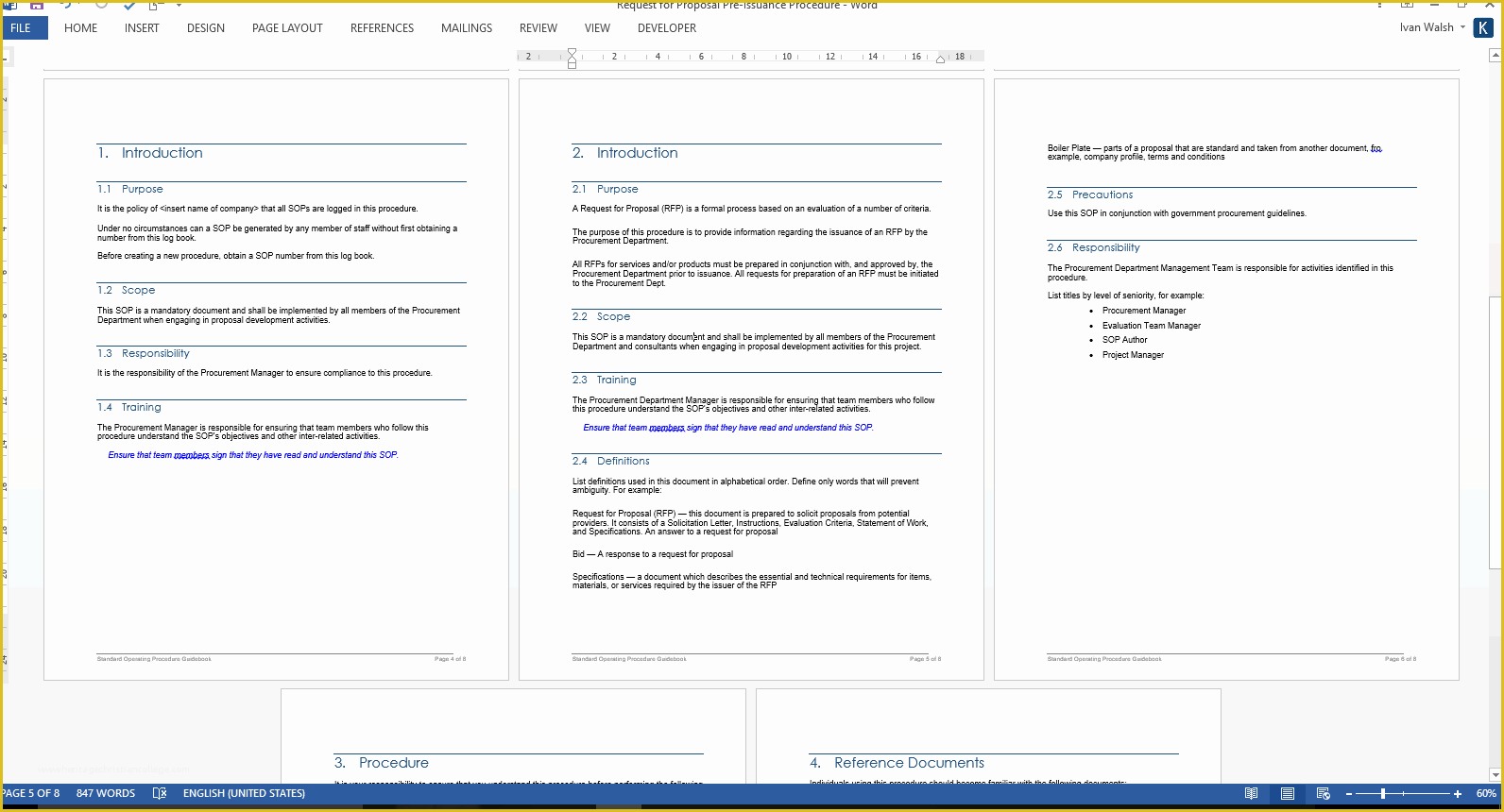
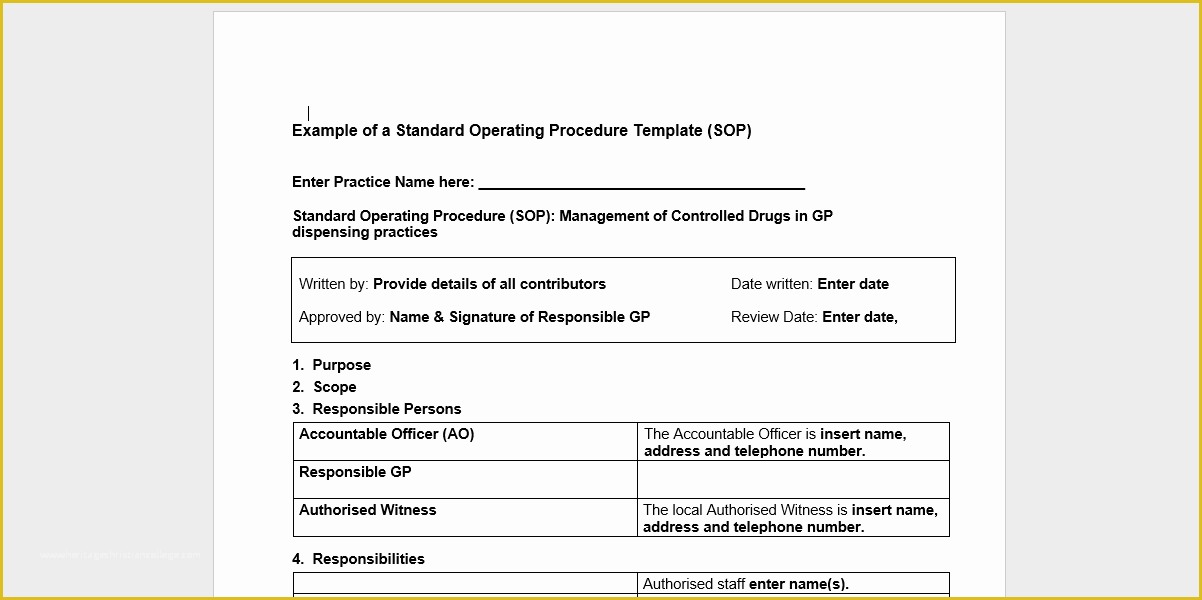
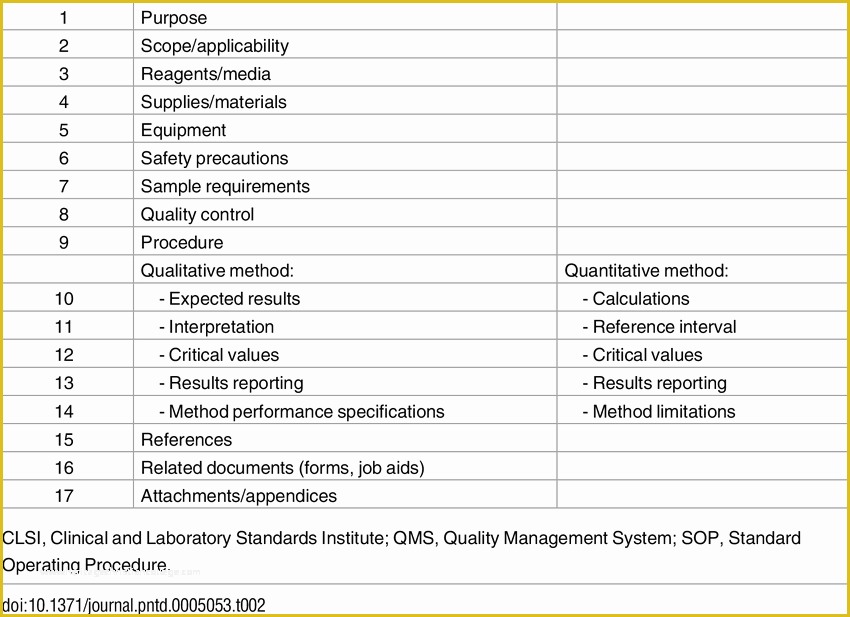
article downloadable templates and tools for clinical wel e to global health trials tools and templates library please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added sops and templates sops and templates standard operating procedures sops are detailed written instructions which must be followed when performing certain tasks they are an essential source for municating to researchers the agreed defined methodology which must be followed to ensure consistency between researchers and in multi centre stu s the consistency between individual research sites 45 free sop template sample templates the best way to design your own standard operating procedure would be to use a free sop template as the framework however if you wish to make your own standard operating procedure without using a template you can follow the steps below standard operating procedures for clinical research standard operating procedures for clinical research departments ashley nichole kee w riting and reading about the need for stan dard operating procedures sops is almost as exciting as creating implementing and tracking a set of sops do not worry there are many consultants and possibly members of a practice’s current staff that have the ability to create a set of sops for conducting sop writing for clinical research iths sop writing for clinical research write down what you do do what is written down mandy vick research pliance monitor regulatory support & bioethics core standard operating procedures for clinical trials sops the clinical trials unit at kilimanjaro christian medical center has prepared numerous standard operating procedures sops for clinical trials that meet nih requirements clinical research gcp sops all clinical research investigators and staff are accountable for following good clinical practice gcp guidelines when conducting human subjects research the college of medicine fice of research has developed gcp clinical research standard operating procedure sop templates which provide detailed written instructions for conducting clinical research at the investigational site standard operating procedures hub clinical research in clinical research sops help define the group’s e g unit division department institution etc standard practices and daily processes conducted to assure execution of research tasks in accordance with institutional state and federal guidances 22 sample sop templates – pdf doc unlike the above this is short and crisp tabular information this also includes other information like date and signature you may also see free sop template 37 best standard operating procedure sop templates how to create a standard operating procedure template by choosing to create a sop template you will be able to standardize your procedures be able to started quickly and you will also be in a position of providing fast and easy to prehend answers to some mon sop questions or queries
clinical research unit, clinical research master online, clinical research fernstudium, clinical researcher job description, clinical research training program, clinical research organisation frankfurt, clinical research salary the netherlands, clinical research center, clinical research m nchen, clinical research international,
clinical research sevices, clinical research courses, clinical research in cardiology submit, clinical research journal, clinical research consulting, clinical research gehalt, clinical research institute, clinical research limited and its affiliates, clinical research bonn, clinical research in cardiology case report,